The Complete ISO 13485 QA Course for Medical Devices
Master ISO 13485: Elevate Medical Device Quality Assurance
4.18 (85 reviews)

7,988
students
9 hours
content
Nov 2023
last update
$19.99
regular price
What you will learn
ISO 13485 Understanding: Gain deep knowledge of ISO 13485 for the medical industry, covering normative references, definitions, and quality management.
Quality Assurance Implementation: Acquire practical skills for implementing quality assurance in medical device manufacturing, ensuring ISO 13485 compliance.
Documentation for Compliance: Learn to create essential ISO 13485 documentation, ensuring compliance through policies, objectives, manuals, procedures, and more
Comprehensive Quality Audits: Master comprehensive quality audits, including matrix tracking and full audits, preparing for ISO 13485 standards alignment.
Why take this course?
🎓 **Course Title:** The Complete ISO 13485 QA Course for Medical Devices
🚀 **Headline:** Master ISO 13485: Elevate Medical Device Quality Assurance
📘 **Description:**
Welcome to **"The Complete ISO 13485 Quality Assurance Course for Medical Devices"** – your definitive guide to mastering the intricacies of quality assurance within the dynamic field of medical devices! This comprehensive course is a treasure trove for both seasoned professionals and those new to the industry, offering a journey from the fundamental principles to advanced practical applications of ISO 13485.
**Key Highlights:**
- **In-Depth Standard Understanding:**
- Gain a profound understanding of ISO 13485, covering normative references, definitions, and critical quality management principles that form the backbone of medical device quality assurance. 🔍
- Dive into the core elements of the standard to ensure you have a solid grasp of its requirements and structure.
- **Practical Implementation Techniques:**
- Acquire practical skills to implement quality assurance in medical device manufacturing. Learn how to ensure compliance with ISO 13485 through structured management, streamlined procedures, and accountability. 🛠️
- Discover actionable steps you can take to align your processes with the stringent requirements of the standard.
- **Effective Documentation and Compliance:**
- Navigate the art of creating essential ISO 13485 documentation, including policies, objectives, manuals, procedures, work instructions, and record-keeping. Ensure effective compliance with industry standards. 📚
- Learn how to develop and maintain documentation that not only meets regulatory requirements but also supports your quality management system.
- **Thorough Quality System Audits:**
- Master the techniques of conducting comprehensive quality system audits! Covering matrix tracking, full audits, and management reviews, you'll be well-prepared for ISO 13485 standards alignment and industry audits! 🔍
- Understand the importance of regular audits in maintaining a robust and effective quality management system.
By the end of this course, you'll not only have a solid theoretical understanding of ISO 13485 but also possess the practical skills needed to implement quality assurance effectively in the challenging landscape of medical device manufacturing.
🎢 **Join us on this educational journey and elevate your expertise in ISO 13485 Quality Assurance!** This course is not just a learning experience – it's a strategic investment in your professional growth, ensuring you stay at the forefront of quality assurance standards for medical devices. Enroll today and step into a world of informed decision-making and enhanced compliance capabilities. Let Petar Dimov guide you through this transformative learning journey! 🌟
Screenshots



Reviews
Veronica
January 10, 2024
The information is very well organized, however, it doesn't have practical cases which can show us examples to apply, I have not see any video in which it is more interactive the information we are receiving , in which I can understand and improve better the application of the ISO.
Andrei
January 10, 2024
Good course, detailed information. It seemed to me that it was too stretched. Much is repeated many times.
Prakash
January 4, 2024
yes it was a good match for me. every detail is explained in a better way.
only half star i cut for the mixing of voice and slides. bcoz everytime there is problem during view that is slide goes to the next but the voice is for previous slide, by which anyone can not concentrate what is to be listen. bcoz if we see something Infront of our eyes and listening something else then its a problem for beginners like me. i hope u understand my this small thing which i want to convey you..( i think this truth no one will tell you)
at last this course have everything about ISO 13485.
great choice for all of you ..
Mohan
November 27, 2023
Provided detailed information. It would be nice if provided with some live examples.. then it would be easy to co-relate with this content.
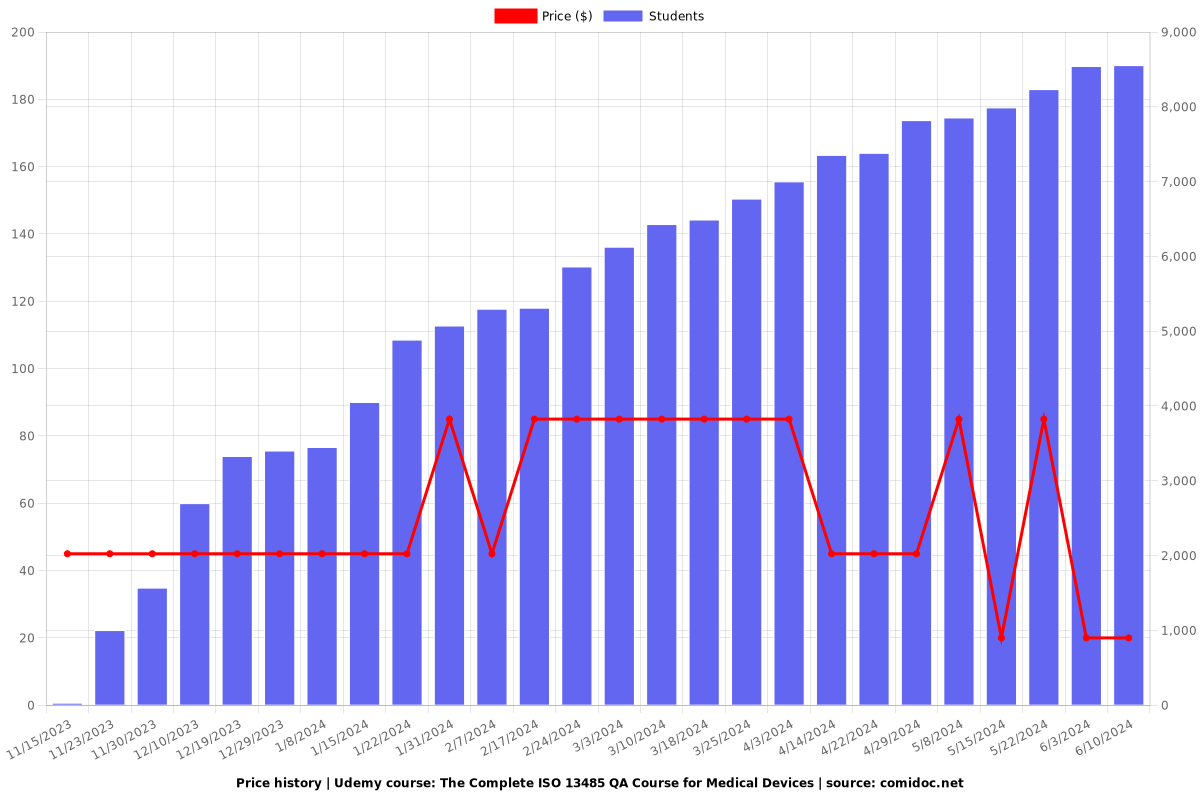
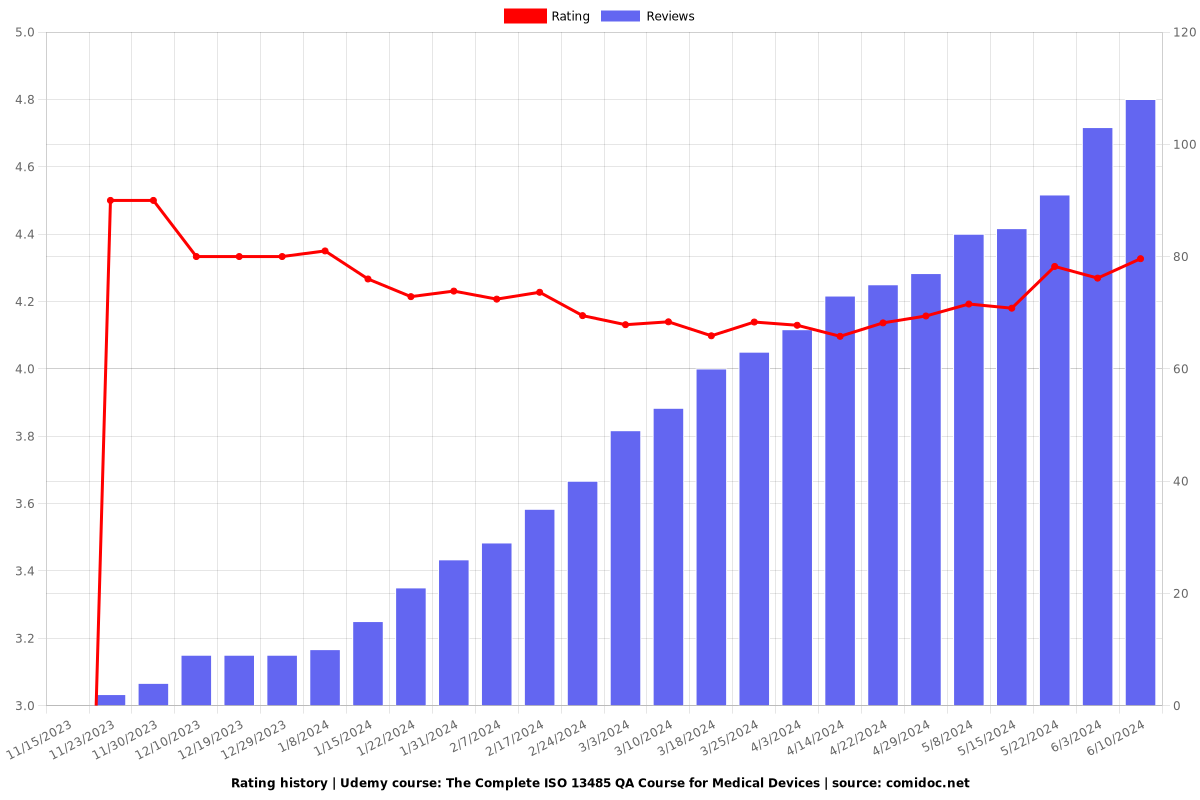
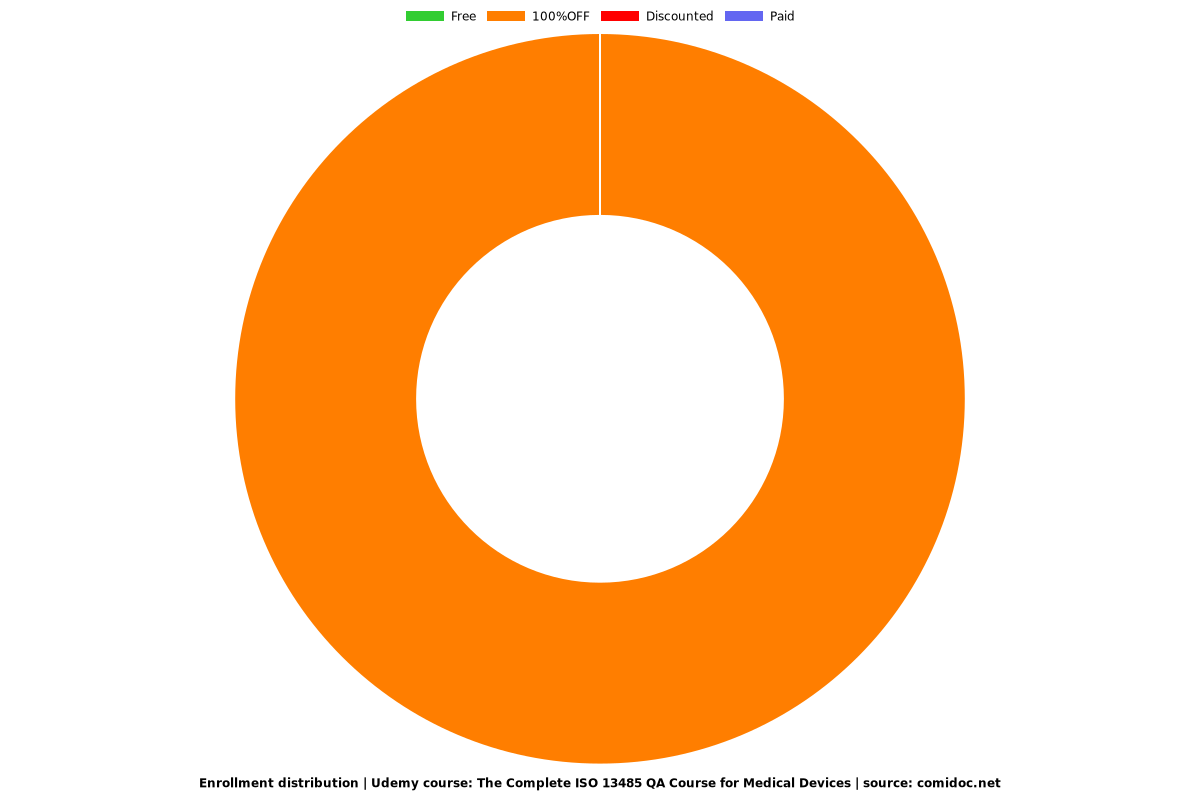
Charts
Price
Rating
Enrollment distribution
5654732
udemy ID
11/11/2023
course created date
11/15/2023
course indexed date
Bot
course submited by